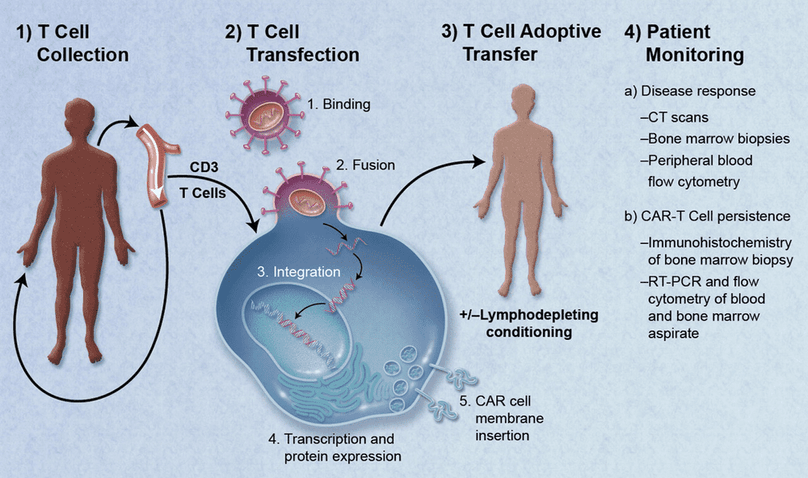
Chimeric Antigen Receptor T Cells (CAR-T Cells) are part of the body’s immune system that has been altered to fight cancer. T cells are taken from a patient then genetically engineered to put CARs on the surface of the cells before multiplying the cells in the lab. After freezing, shipping, and thawing, they are reintroduced into the patient’s bloodstream. This process is called immunotherapy or, specifically, CAR-T therapy.
Types of Cancer Treated
One of the emerging adoptive cell transfer (ACT) immunotherapies, CAR-T therapy has been clinically developed more extensively than TILs or TCRs. These cells are considered a living drug that may also prevent reoccurrence of cancers like some adult lymphomas and acute lymphoblastic leukemia in children and young adults.
FDA Approved Treatments
CAR-T therapy is FDA approved in the following trademarks:
- Tisagenlecleucel - Trademarked Kymriah
- Axicabtagene ciloleucel – Trademarked Yescarta
- Tocilizumab – Trademarked Actemra
Tisagenlecleucel has been approved for patients up to 25 years old with some cases of acute lymphoblastic leukemia. Axicabtagene ciloleucel is approved for adults with certain large B-cell lymphomas. In young children two and older up to adults, Tocilizumab is approved to treat cytokine release syndrome (CRS) induced by CAR-T cells.
Possible Side Effects
While potentially life-saving, there are also side effects to treatment using CAR-T cells.
- Cytokine release syndrome produces flu-like symptoms
- Neurologic events like brain disease or injury, confusion and seizures
- Low white blood cells (neutropenia)
- Low red blood cells (anemia)
Most of these side effects can be treated with pharmaceuticals or can be allowed to abate on their own. However, the benefits can far outweigh potential side effects when the disease being treated is life-threatening.
Genemod is a user-friendly software that allows researchers to get up and running quickly with little learning curve. For more information on how their software can help with sequence editing, digest mapping and more, contact Genemod for a demo.